Brief version of the antibody test "instruction manual"
In the last article, we talked about how to test for antigens at home. This time, we will talk about the antibody test, which is also a novel coronavirus test method but not well-known.
Antibody (Antibody), also known as Immunoglobulin (Ig), is a class of miniature proteins produced in the human body due to the stimulation of a variety of different types of pathogens. It often has the property of producing special reactions to corresponding pathogens. According to their molecular structure and antigen specificity, immunoglobulins can be divided into IgG, IgM, IgA, IgD and IgE.
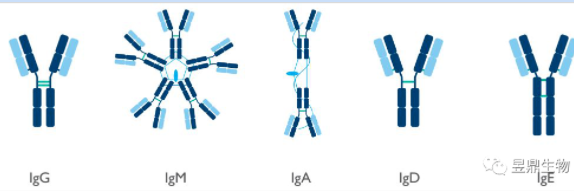
Figure 1 Types of immunoglobulins
(Photo source: Proteintech Experimental Technical Manual)
IgA mainly plays its function in secretory fluid and is the main antibody of mucosal local anti-infection immunity. The content of IgD in serum is small and the function is not very clear. IgE is the Ig with the lowest serum content, which can cause type I hypersensitivity reaction. IgG is the main antibody in the secondary immune response of the body, and its increased content generally indicates past pathogen infection or chronic infection. IgM is the first antibody to appear in the initial immune response, and elevated levels usually indicate the invasion of a pathogen. Therefore, IgG and IgM antibodies can be used as two important targets to detect pathogen invasion and immune system response.
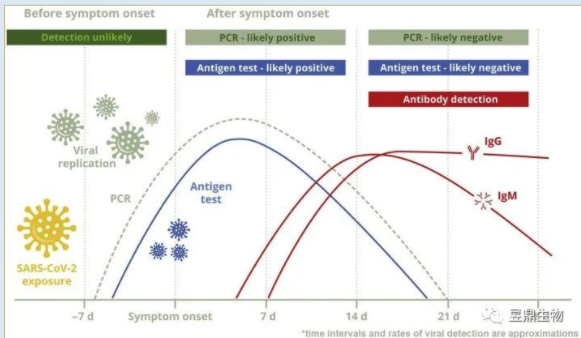
Figure 2 Antibody production cycle curve in the body
(source: doi: 10.1016 / j.j aph. 2021.06.012. Epub 2021 Jun 12.)
After being infected with the novel coronavirus, the symptoms of many small partners basically disappeared a few days after taking the medicine, and the test results also showed negative. After two days of examination, they returned to positive. But many studies have pointed out that when we have symptoms for about 2 days, antibodies have been produced, otherwise antigen detection can not turn negative. It's just that our bodies first produce IgM, a short-acting antibody, and then IgG, a long-acting antibody, which takes about two weeks to reach its higher levels.
Detection principle
So how do you test for COVID-specific IgM and IgG levels? The main technique is colloidal gold immunochromatography.
First, three capture antibodies, anti-human IgM antibody, anti-human IgG antibody and anti-rabbit IgG antibody, were fixed on the nitrate cellulose membrane as IgM detection line (M line), IgG detection line (G line) and quality control line (C line). The gold particles were then coupled with the S protein of the novel coronavirus by colloidal gold technology, and the gold particles were connected with the rabbit IgG, and the two were sprayed and fixed on the binding pad.
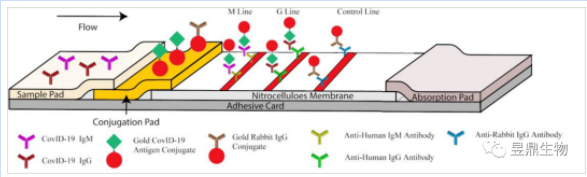
Figure 3 Principle of antibody test kit
When the sample droplets are added to the sample pad, they flow towards the binding pad under the action of capillary, dissolve the gold label antigen fixed on the binding pad, and react with it to form the target antibody-antigen-gold particle complex. Subsequently, when the solution to be tested reaches the detection area, the specific trapping antibody on the M and G lines will bind specifically to the target - colloidal gold complex again, and the complex will be trapped on the M or G lines. When a large amount of complex is trapped and aggregated, it will show visible red or pink spots. Therefore, the color changes of M and G lines can be used to determine whether the solution contains anything to be measured.
Result interpretation
When only line C appears red bands, it indicates that the sample does not contain the substance to be tested, and the result is negative. When lines C and M show uniform red bands, it indicates that the sample contains anti-COVID-19 IgM and that the subject's immune system is actively producing antibodies to the recently infected virus. When lines C, M, and G all show red bands, the sample contains both anti-COVID-19 IgM and anti-COVID-19 IgG, and the subject's immune system actively produces antibodies to the ongoing infection, which may have started 14 days ago. When the C and G lines show red bands, the sample contains IgG of the novel coronavirus, and the subject's immune system has produced antibodies against the target virus. When the three lines do not show color, it indicates that the reagent is invalid and the test result is invalid.
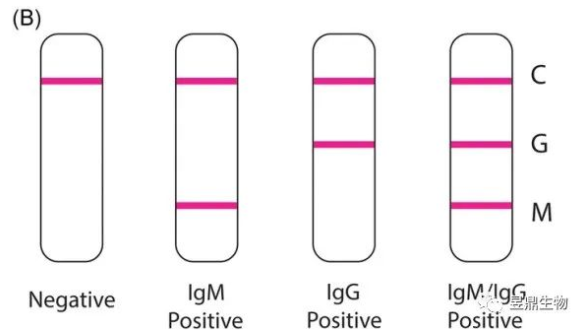
Figure 4 Interpretation of antibody detection results
(Source: doi: 10.1002/ jmv.25727.Epub 2020 Apr 13.)
Due to the uniform existence of antibodies in the blood, no matter by collecting fingertip blood, venous blood or other parts of the blood, the test results have little difference, which greatly avoids the false negative situation that may occur easily in the initial infection of nucleic acid test due to the small number of upper respiratory viruses, and the operation is convenient and the test time is short. However, in the incubation period or the early stage of infection, the immune system has not produced enough antibodies, antibody detection reagents basically can not play a role; At the same time, the presence of antibodies in relatively cured patients will cause interference to the detection results of a large area of the population.
Therefore, antibody test kit can be used as an effective supplement to nucleic acid test, rather than a substitute for nucleic acid test. Only by complementing each other and combining advantage points can "false negative" and "false positive" be avoided with greater probability and the real results closest to the facts be obtained.
Just because we're healthy and protected from Omicron for a while doesn't mean we can go easy on ourselves. After recovery, you still need to wear a good mask, frequent ventilation, frequent hand washing and disinfection. Of course, I hope you will be disease-free and healthy in the New Year!
References:

