FISH test-noninvasive and efficient, help you find early bladder cancer
In the article "FISH- the" FISH "of Pathology Department, fluorescent in situ hybridization", we already know that fluorescent in situ hybridization is a combination of cytogenetics and molecular biology technology developed in the late 20th century, and has realized its value in many fields. This article will take you to have an in-depth understanding of the application of FISH detection in bladder cancer.
In 2020, there were 573,000 cases of bladder cancer and 213,000 deaths worldwide, ranking the 10th in the global incidence of cancer [1]. In 2020, 86,000 new cases of bladder cancer were reported in China, accounting for 14.9% of new cases and 39,000 deaths, accounting for 18.5% of global deaths from bladder cancer. Both new cases and deaths are the highest in the world. In the next 5 years, the number of new cases and deaths of bladder cancer in China will continue to increase. With the aging of the population, the number of new cases and deaths of bladder cancer in China are expected to exceed 100,000 and 48,000 respectively in the next 5 years [2].
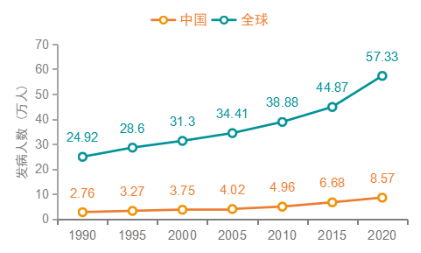
Figure 1 Trends in the incidence of bladder cancer in China and globally from 1990 to 2020
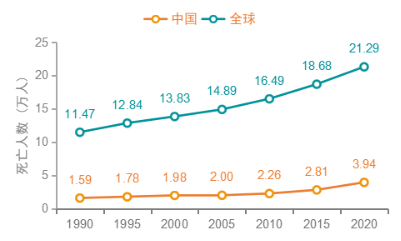
Figure 2 Trends in bladder cancer deaths in China and globally, 1990-2020
As one of the most common malignant tumors in the urinary system, bladder cancer is usually presented as painless, intermittent, gross hematuria, and sometimes microscopic hematuria. About 75% of newly diagnosed urothelial neoplasms of the bladder are superficial bladder neoplasms with a good prognosis. However, the recurrence rate of bladder tumors is very high in clinic. Data [3] show that about 60% ~ 85% of bladder tumors will relapse within 2 years after surgery, and about 15% ~ 23% of recurrent bladder tumors will progress into invasive bladder tumors. Once invasive tumors develop, they can seriously affect the prognosis. If diagnosed early, bladder cancer has a survival rate of up to 96% after treatment.
Currently, the main diagnostic methods for bladder cancer include cytology and cystoscopy. The former is of low sensitivity, which affects the use value, while the latter is invasive examination, which brings great pain to patients. Therefore, it is necessary to develop a non-invasive, highly sensitive and highly specific detection method for early bladder tumor recurrence. Sandberg[4] believed that bladder cancer is prone to abnormal structure and number of chromosomes. Current studies have confirmed that genetic changes of chromosomes 3, 7, p16 and 17 are closely related to the early occurrence, development and recurrence of bladder cancer. Therefore, the use of this non-invasive, highly sensitive and highly specific FISH technique to detect the aberrant chromosome of exfoliated cells in urine specimens is of great significance for the early diagnosis and prognosis assessment of bladder cancer.
The chromosome and gene abnormality detection kit, developed by Yuting, established the chromosome 3, Chromosome 7, Chromosome 17 and chromosome p16 chromosome specific probe (Chromosome 3, Chromosome 7, Chromosome 17) and gene located probe (gene located probe). GLP) FISH probe combination, using FISH technology, through the detection of urine DNA sequence changes, for the early diagnosis of bladder cancer. On May 30, 2022, the Bladder cancer cell chromosome and Gene abnormality detection kit (in situ Hybridization) developed by Yuding Biotechnology was approved by the National Medical Products Administration (NMPA) and put on the market (record No. : ZhehangShibei 20190440). At present, it has been sold in hospitals as an early bladder cancer diagnosis product.
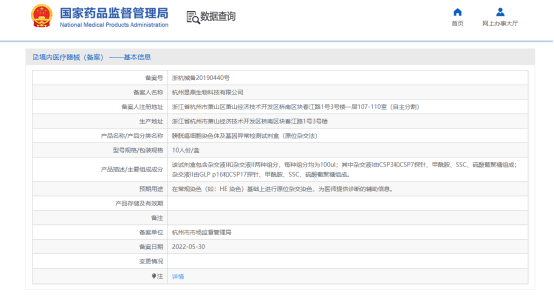
Figure 3 Yuding Biometrum ™- Bladder Cancer cell chromosome and genetic abnormality detection kit has been certified
According to the clinical trial center, the product has high efficacy in the diagnosis of early bladder cancer, with sensitivity greater than 80% and specificity greater than 95%.
Compared with the currently commonly used diagnostic methods for bladder cancer in clinical practice, the Blang Easy Test ™ -bladder cancer cell chromosome and gene abnormality detection kit developed by YudingBio has obvious advantages, taking into account both high sensitivity and specificity. Through the gene detection of urine shedding cells, non-invasive sampling is achieved, which increases the compliance of patients to be tested and reduces unnecessary invasive examination. Therefore, it has a positive impact on the clinical diagnosis and treatment decision of bladder cancer, and has a high clinical promotion value. It is a breakthrough non-invasive test means after cystoscopy, biopsy and urocytology, and provides a new weapon for the clinical precision diagnosis and treatment of bladder cancer.
Yuding will always focus on the field of non-invasive cancer diagnosis, hoping to provide complete solutions for the early diagnosis and treatment of common cancers in China, as well as relapse monitoring.
References:
[1] Sung H, Ferlay J, Siegel R L, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2021 states (3) : 209-249.
[2] Global Cancer Obserbatory, GLOBOCAN 2020, Estimated age-standardized incidence and motality rates in 2020, males, age 50+. Available from: https://gco.iarc.fr/today/online-analysis.
[3] Liu Yuexin, Liu Xiaochao, Kang Wenting, Chen Shan, Wang Wei, Yan Wei. Clinical application of fluorescence in situ hybridization in bladder cancer detection [J]. Beijing Med,2013,35(01):1-3.DOI:10.15932/ J.0253-9713.2013.01.027.
[4] Sandberg AA,Berger CS. Review of chromosome studies in urological tumours.Ⅱ. Cytogenetics and molecular genetics of bladder cancer[J].J Urol,1994,151(3) : 545-560. (in Chinese)

