Liquid biopsy- - - -a dawn of early tumor diagnosis
Liquid biopsy---- The dawn of early diagnosis of cancer
Cancer is one of the most common diseases found in human history, and traditional tissue biopsies that improve the prognosis of cancer are invasive procedures that are performed after a tumor is found, often at an advanced stage of the cancer when it would be more difficult for us to treat it. The early detection of cancer has become a huge problem.
Liquid biopsies are at the forefront of early cancer detection because they require only a sample of body fluid, are simple, quick, and less invasive. In this paper, we will discuss the principles, advantages and applications of liquid biopsy in cancer diagnosis and surveillance.
What is a liquid biopsy?
Liquid biopsies, or circulating tumor omics, are an innovative field in precision oncology and emerging techniques that can overcome the limitations of current tissue biopsies. Liquid biopsy can diagnose diseases by sampling cerebrospinal fluid, saliva, pleural fluid, blood, ascites, urine and other liquid samples, which can avoid the influence of tissue heterogeneity on tumor molecular typing to a certain extent.
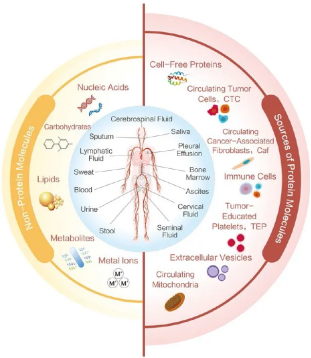
figure1 Schematic diagram of categories of liquid biopsy and the range of body fluids that can be detected
At present, blood based liquid biopsy is the most important research direction. analyte that can be detected in peripheral blood includes circulating tumor protein, circulating tumor cell (CTCs), circulating cell-free DNA(cfDNA) and circulating tumor DNA(ctDNA). Other analytes included circulating cell-free Rnas (Cfrnas), extracellular vesicles (EVs) and their components, exosomes (exosomes), and tumor education platelets (TEPs). Each component provides a variety of biological information and can also be used directly or indirectly as a tumor biomarker for liquid biopsies.
What are the advantages of liquid biopsy?
Compared with tissue biopsy, liquid biopsy technology has the advantages of non-invasive, strong repeatability, early diagnosis, dynamic monitoring, and overcoming tumor heterogeneity.
>>>> Noninvasive
Tissue biopsy usually requires surgical means to obtain tissue, and there may be risks of bleeding, pain, infection, and tumor dissemination. In addition, tissue biopsy risk is greater for some patients with special or intolerable locations. The most obvious advantage of liquid biopsy over tissue biopsy, is non-invasive, low location requirements, high operability, less pain, and higher patient acceptance
>>>> Found a tumor tissue that is undetectable by clinical means
Tissue biopsy is mainly dependent on imaging examination, and it has no mass effect on imaging during the early tumor stage or postoperative tumor-free period. However, there may be some tumor-related information in the blood or other body fluids, such as DNA fragments or exosomes released by tumor cells. Early tumor screening and postoperative recurrence risk prediction can be achieved through liquid biopsy.
table1. Comparison between traditional screening techniques and liquid biopsy techniques

>>>>Real-time effect of a liquid biopsy
Liquid biopsy markers have a very short half-life and more timeliness. Studies have shown that the half-life of ctDNA is less than 2 hours, which allows it to accurately reflect real-time tumor information and achieve the purpose of guiding medication, efficacy and drug resistance monitoring. Studies have shown that the average time consuming of liquid biopsy is 9 days and tissue biopsy is 15 days, suggesting that liquid biopsy can provide effective clinical information earlier.
>>>>Implement dynamic monitoring
Because of its flexible and non-invasive characteristics, the liquid biopsy can realize the dynamic monitoring of recurrence, efficacy or drug resistance. Imaging tips often lag behind, and can only show the change of tumor morphology, and cannot analyze the fluctuation of different subclones, and frequent detection also has the harm caused by radiation.
>>>>Overcoming the tumor heterogeneity
Due to the strong heterogeneity of tumors, the genetic information of different individuals, different tumors of the same individual, different subclonal tissues of the same individual, different cells of the same site, and different cells of the same subclone can be different. Traditional tissue biopsy, which is a part of the diseased tissue for testing, has limitations. Liquid biopsy was taken from total circulating DNA molecules, theoretically more comprehensive and lower heterogeneous bias. If tissue biopsy and liquid biopsy are combined, the positive rate can be further improved and more patients can benefit.
What are the applications of liquid biopsy?
Liquid biopsies have been widely evaluated as potential biomarkers for effective disease management in several areas:(1) Detection of MRD after surgery or adjuvant therapy; (2) Continuous monitoring of treatment response and evaluation of drug resistance mechanisms (3) Targeted therapy selection based on tumor mutant spectrum; (4) Precancerous screening for early disease and identification of tumor origin.
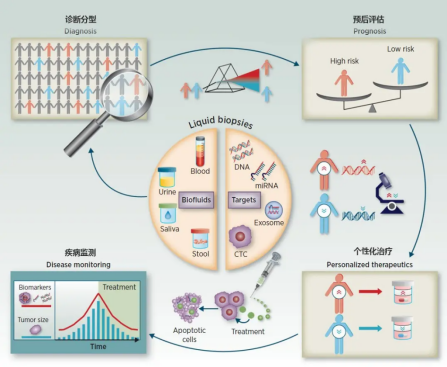
figure2. Application of liquid biopsy
Source:2017 American Association for Cancer Research
Detection of minimal residual lesions (MRDS) after surgery or adjuvant therapy
MRDS are the small number of cancer cells that remain in the body after surgery and treatment, below the detection limit of routine imaging and pathological examination. Currently, a clinical diagnosis of recurrence or metastasis can only be confirmed if the tumor size is within the detectable range of imaging. Liquid biopsies have the potential to detect tiny amounts of cancer cells, leading to earlier treatment decisions when tumors are smaller.
Detection of minimal residual lesions (MRDS) after surgery or adjuvant therapy
In addition to assessing the risk of recurrence by assessing MRDS after surgery or chemotherapy, liquid biopsies can also be used to monitor the response of advanced or metastatic cancers to systemic therapy, supplementing the diagnostic role of serum biomarkers and imaging in a non-invasive manner.
Liquid biopsy was used to select targeted therapies for tumor mutations
The appeal of liquid biopsies is that tumor mutation analysis is no longer limited by biopsy anatomical site, but covers the entire tumor spectrum of a particular patient. This raises the possibility of using liquid biopsies to guide treatment decisions for patients with advanced cancer. Currently, the U.S. Food and Drug Administration (FDA) has approved 43 companion diagnostic devices, 11 of which are liquid biopsy-based tests.
Precancerous screening for early disease and identification of tumor origin
Malignant tumor is a heterogeneous disease, and liquid biopsy can reflect the whole genomic picture of the tumor, which can not only reduce the deviation caused by tumor heterogeneity in diagnosis, but also reflect the dynamic changes of tumor development in time. A large number of studies and practices have shown that the application of tumor molecular markers to detect mutations, deletions, rearrangements, methylation, amplification and insertion in the genome of tumor cells at the early stage of tumor (stage I and stage II) can effectively prolong the overall survival of tumor patients and reduce the economic burden of disease.

